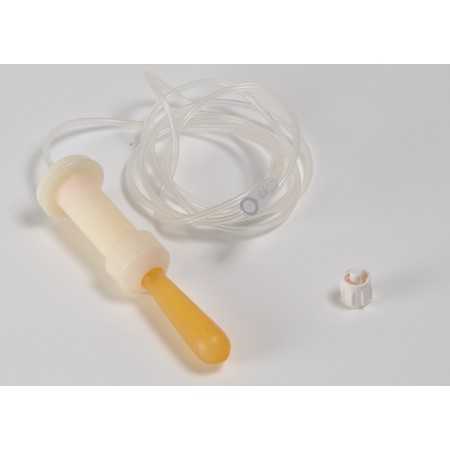
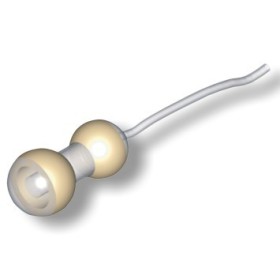
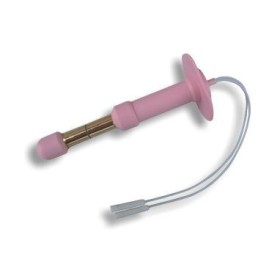
PERIPROBE ANA-BFT - Anal Probe for Perineal Re-education by Manometric Biofeedback
by credit card, Paypal and bank transfer.
with BRT, DPD and FedEx couriers
via Whatsapp + 39.371.43.61.201
PERIPROBE ANA-BFT -Anal Probe for Perineal Re-education by Manometric Biofeedback
Single-patient anal probe for incontinence re-education by anal manometric biofeedback.
Thanks to the shape, specifically designed for manometric recording, depending on the anal morphology, the probe remains perfectly in position, allowing reproducible recordings and limiting artifacts.
Due to its particular design, no pre-inflation phase of the balloon is necessary; The only precaution is to connect the probe to the device before introducing the balloon into the anal orifice. Avoid storing the probe exposed to air and sunlight which would lead to rapid aging of the rubber latex.
The probe must be used with equipment designed for manometric measurements, using standard luer-lock pneumatic connections (ISO 594).
Position of use
Suitable for couch therapy.
Specifications:
• Patient contact materials: ABS + Rubber latex.
• Invasiveness: anal orifice for a period of up to 1 hour.
• Dimensions: ∅ min/max. 8/12 mm, Length 100 mm.
• Weight: 15 g.
• Available colors: White.
• Packaging: Supplied in a single package (non-sterile) with warranty label and notes of use.
• Cleaning and disinfection: with water and possibly mild soap. Disinfect with alcohol if necessary and rinse with water before use. DO NOT sterilize.
• Personal use: Can be used for 60 sessions by a single patient (cannot be used by multiple patients)
• Combined use: It must be used with a medical electrical device (with CE marking) for manometric biofeedback and luer-lock pressure inlet (ISO 594) capable of detecting pressures from 0 to 300 cmH2O.
• Production quality control: In certified ISO9001-ISO13485 system.
CONTRAINDICATIONS - Anal infections, - Do not use on subjects allergic to rubber latex.
Use and storage environment: +5 °C to + 35 °C with U.R. between 20% and 80%.
CE marking: Class IIa device according to regulation 5 Annex IX 93/42/EEC - Marking CE0051, issued by IMQ, according to Annex II of Directive 93/42/EEC.
Registration in the Repertoire of Medical Devices of the Ministry of Health
NDT: U070399
BD/RDM : 787075
Ean: