Security policy
(edit with the Customer Reassurance module)
(edit with the Customer Reassurance module)
Delivery policy
(edit with the Customer Reassurance module)
(edit with the Customer Reassurance module)
Return policy
(edit with the Customer Reassurance module)
(edit with the Customer Reassurance module)
Description
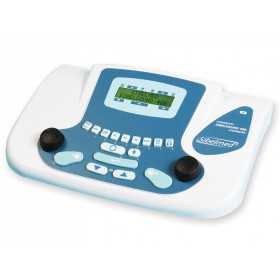
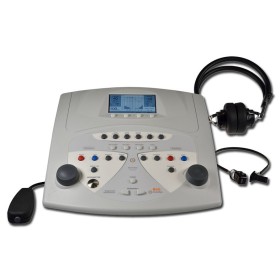
MAICO MA1 AUDIOMETER WITH AUDIOMETRIC HEADPHONES
Screening audiometer with a super-lightweight design, small enough to fit in the palm of your hand.
Ideal for rapid screening purposes.
The device is capable of transmitting a pure tone to only one ear at a time.
The stimulation intensity range is 15 to 50 dB HL in 5 dB level increments.
The test frequencies range from 500 to 4000 Hz.
Automatic ON/OFF function.
Supplied with DD45 audiometric headphones and 2 AA batteries.
Multilingual manual: GB, FR, IT.
1 pc.
Specifications:
Test Signal: Pure Tone (Continuous)
Test frequencies: 500, 1000, 2000, 4000 Hz
Level Increments: 5 dB
Stimulus intensity: 15 dB HL - 50 dB HL
Accuracy: ± 4 dB
Distortion: less than 3%
Dimensions: 6.4x15.3xh 2.3 cm
Weight: 115 g
Power supply: 2 x AA batteries (included)
Standard: IEC 60601-1:2012 Type B, IEC60601-1-2:2014
Classification: Class IIa (EU 2017/745)
Medical device
Screening audiometer with a super-lightweight design, small enough to fit in the palm of your hand.
Ideal for rapid screening purposes.
The device is capable of transmitting a pure tone to only one ear at a time.
The stimulation intensity range is 15 to 50 dB HL in 5 dB level increments.
The test frequencies range from 500 to 4000 Hz.
Automatic ON/OFF function.
Supplied with DD45 audiometric headphones and 2 AA batteries.
Multilingual manual: GB, FR, IT.
1 pc.
Specifications:
Test Signal: Pure Tone (Continuous)
Test frequencies: 500, 1000, 2000, 4000 Hz
Level Increments: 5 dB
Stimulus intensity: 15 dB HL - 50 dB HL
Accuracy: ± 4 dB
Distortion: less than 3%
Dimensions: 6.4x15.3xh 2.3 cm
Weight: 115 g
Power supply: 2 x AA batteries (included)
Standard: IEC 60601-1:2012 Type B, IEC60601-1-2:2014
Classification: Class IIa (EU 2017/745)
Medical device
Data sheet
6 other products in the same category: