BD Venflon Pro Safety cannula needle 22G 0.9x25 mm sterile - pack. 50 pcs.
- Over 90,000 satisfied customers since 2020
- Secure payments: credit card, PayPal and bank transfer
- Expedited shipping: Poste, BRT, FedEx
- Customer support Whatsapp +39 371 43 61 201
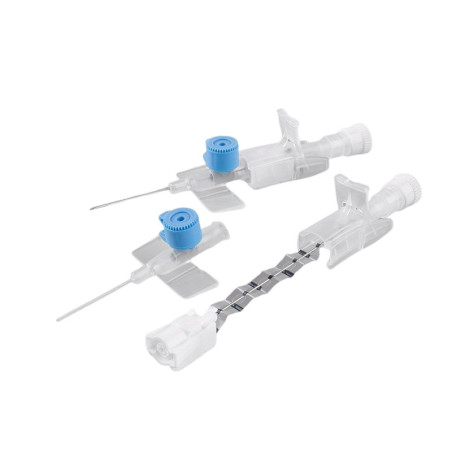
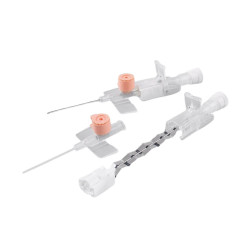
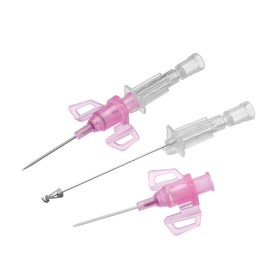
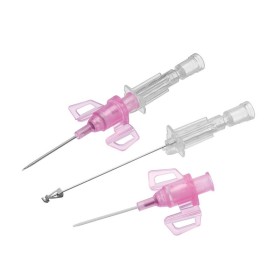
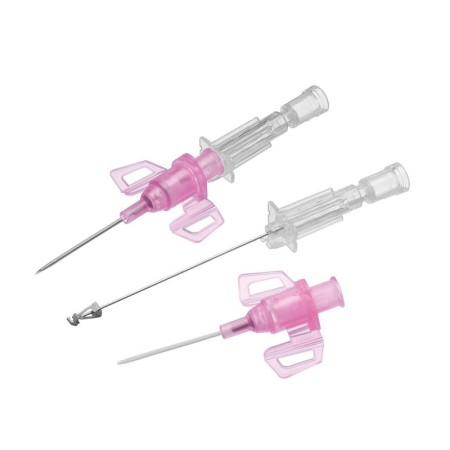
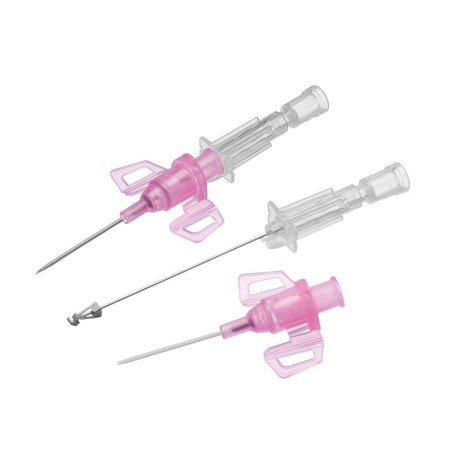
The BD Venflon Pro Safety 22G 25mm sterile cannula needle - pack of 50 is a single-use, sterile, and pyrogen-free medical device designed for short-term peripheral venous cannulation. Manufactured by BD (Becton Dickinson) , it is widely used in hospital and outpatient settings to ensure high standards of safety, clinical reliability, and patient comfort.
Product code 393222 identifies a 22G cannula needle with a 25 mm catheter length , particularly suitable for adult patients with small - medium-sized veins and for standard intravenous therapies. The catheter is made of BD Vialon polyurethane , a proprietary biomaterial clinically tested to reduce the risk of phlebitis and infiltration, improving tolerability and extending dwell times compared to traditional materials.
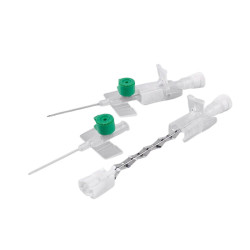
The device features a passive and irreversible safety system that, once the needle is removed, completely covers the cutting edge, significantly reducing the risk of accidental needle sticks. The integrated injection valve , equipped with an anti-reflux mechanism and color coding, provides a safe and easily identifiable second access point. The transparent reflux chamber and Luer - Lock cone facilitate correct positioning and visual control during insertion.
The BD Venflon Pro Safety 393222 model is power injectable , compatible with the use of high pressure contrast media up to 325 PSI , making it also suitable for diagnostic procedures with automatic injectors. The maximum injection speeds are:
- 7 ml/s for fluids with viscosity < 11.8 cP
- 5 ml/s for fluids with viscosity ≤ 27.5 cP
The gravity flow rate is 42 ml/min . The device is latex-free , PVC-free , and DEHP-free , for greater safety even for sensitive patients.
Classified as a Class IIa medical device according to the MDR Regulation (EU) 2017/745, it complies with the main international standards, including ISO 10555 - 5 and ISO 23908 .
The package contains 50 pieces , ideal for supplying hospital departments, clinics and healthcare facilities that require a reliable, safe and high-performance device, even in the diagnostic field.
Attachments
Scheda Tecnica (IT)
BD Venflon Pro Safety cannula needle 22G 0.9x25 mm sterile - pack. 50 pcs.
- Over 90,000 satisfied customers since 2020
- Secure payments: credit card, PayPal and bank transfer
- Fast shipping: Post and BRT
- Customer support Whatsapp +39 371 43 61 201
Other similar products:

Your review appreciation cannot be sent
Report comment
Report sent
Your report cannot be sent
Write your review
Review sent
Your review cannot be sent