Sigurna plaćanja
kreditnom karticom, Paypalom i bankovnim transferom.
kreditnom karticom, Paypalom i bankovnim transferom.
Brza dostava
s BRT, DPD i FedEx kuririma
s BRT, DPD i FedEx kuririma
Služba za korisnike
putem Whatsappa + 39.371.43.61.201
putem Whatsappa + 39.371.43.61.201
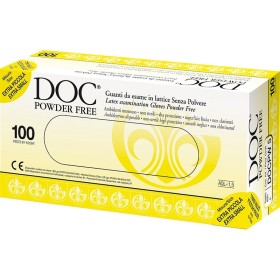
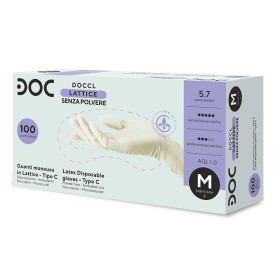
Opis
Tehnički list
6 ostale proizvode u istoj kategoriji: