by credit card, Paypal and bank transfer.
with BRT, DPD and FedEx couriers
via Whatsapp + 39.371.43.61.201
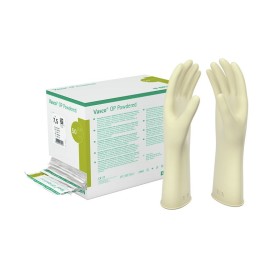
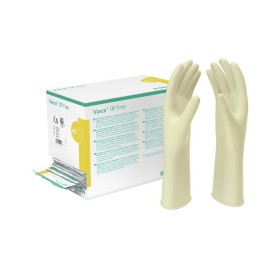
Medical glove in NATURAL RUBBER LATEX, lubricated with powder with performance requirements in compliance with the regulations relating to Class Ia Medical Devices, with high sensitivity and comfortable use. Non-sterile, natural rubber latex glove, ambidextrous, with piping, with matt internal/external finish, internally lubricated with bio-absorbable vegetable powder compliant with the US pharmacopoeia (internal 100 mg/external 50 mg +/- 25%).
Class I Medical Device pursuant to Regulation (EU) 2017/745. National Classification of Medical Devices (NDT) pursuant to D.M. Ministry of Health of 8 June 2016: Cod. T01020201. D.M. registered in the Repertoire of Medical Devices pursuant to D.M. Ministry of Health of 20.2.2007 and subsequent amendments. Complies with EN 455 1,2,3,4 standards.
Type B protective gloves against chemicals and microorganisms, to be used for activities falling under risk category III (Regulation (EU) 2016/425). Complies with EN 420 and EN 374 standards.
Type C protective gloves against chemicals and microorganisms, to be used for activities falling under risk category III (Regulation (EU) 2016/425). Complies with EN 420 and EN 374 standards.
Use
Multipurpose disposable: examination, therapy, diagnostics, laboratory, ward.
Performance
AQLProduct in ISO 9001:2015 and ISO 13485:2016 certified facilities.
Specifications:
- Color: white/latex
- Length: 240 mm (minimum value)
- Thickness: 0.10 mm (average value)
- External finish: smooth
- Sizes: S 6/6,5 – M 7/7,5 – L 8/8,5
- Packaging: packed in dispenser box of 100 pcs